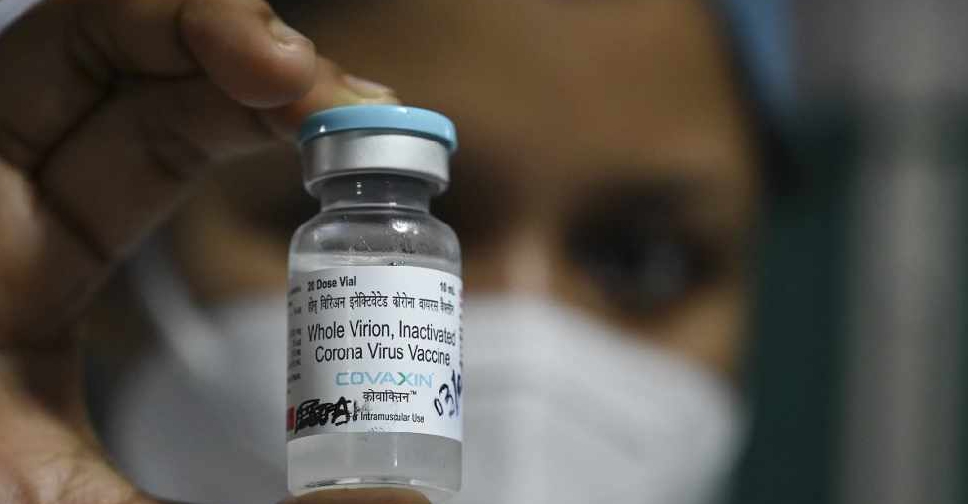
Bharat Biotech's homegrown COVID-19 vaccine has shown an interim vaccine efficacy of 81% in late-stage clinical trials, the Indian company said on Wednesday.
The interim analysis was based on 43 recorded cases of COVID-19 in the trial of 25,800 participants, conducted in partnership with the Indian government's medical research body.
Thirty-six of the 43 cases were recorded in participants who received a placebo, compared with seven cases in people who were given the Bharat Biotech vaccine, pointing to an efficacy rate of 80.6%, the company said.
India had approved the vaccine, branded COVAXIN, in January without late-stage efficacy data, raising questions about its effectiveness.
India's vaccination drive, currently underway, includes COVAXIN and a vaccine developed by Oxford University and AstraZeneca.
Earlier this week, Indian Prime Minister Narendra Modi was inoculated with the first dose of COVAXIN.



 US Supreme Court strikes down Trump's global tariffs
US Supreme Court strikes down Trump's global tariffs
 Police search royal mansion as investigation into king's brother goes on
Police search royal mansion as investigation into king's brother goes on
 7 bodies found after Chinese tour bus plunges into frozen lake in Russia's Siberia
7 bodies found after Chinese tour bus plunges into frozen lake in Russia's Siberia
 ASOS co-founder Quentin Griffiths dies in Thailand after balcony fall
ASOS co-founder Quentin Griffiths dies in Thailand after balcony fall



