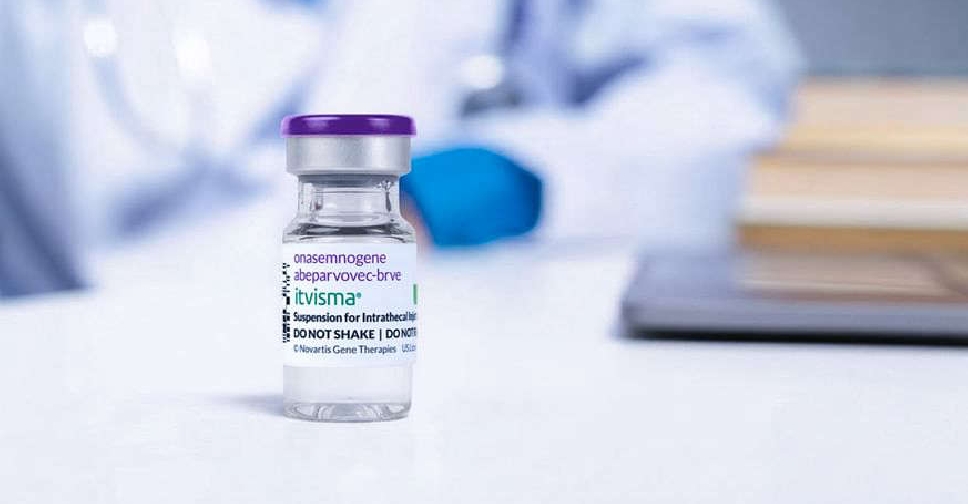
Abu Dhabi has become the first in the world to deliver Itvisma, a one-time gene therapy specifically designed to target the underlying genetic cause of spinal muscular atrophy (SMA) in patients aged two years and above.
Sheikh Khalifa Medical City (SKMC) has successfully administered this groundbreaking gene therapy, developed by Novartis, under the supervision of the Department of Health – Abu Dhabi (DoH), the regulator of the healthcare sector in the emirate.
The therapy had been previously approved by the UAE on November 25, positioning it among the first countries globally, after the USA, to endorse this pioneering treatment.
Designed for simplicity long-term impact, Itvisma (onasemnogene abeparvovec) replaces the missing SMN1 gene to improve motor function, reducing the need for continuous treatments required by other therapies.
Dr. Noura Khamis Al Ghaithi, Under-Secretary of DoH, stated that “this milestone reflects Abu Dhabi’s commitment to delivering world class care and strengthening its position as a global leader in healthcare driven by genomics and precision medicine."



 UAE pledges $8 million grant for refugees in East Africa
UAE pledges $8 million grant for refugees in East Africa
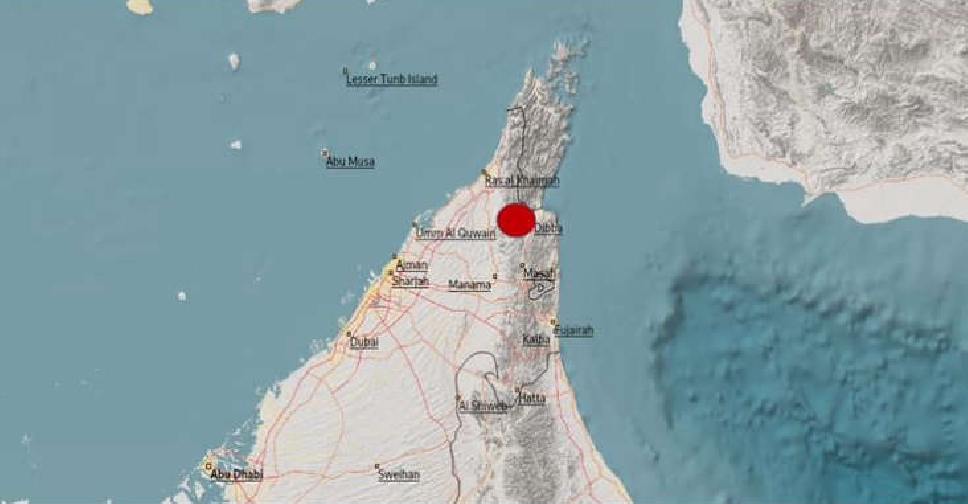 Magnitude 2.9 earthquake recorded in southern Musandam
Magnitude 2.9 earthquake recorded in southern Musandam
 UAE welcomes UN assessment mission to El Fasher
UAE welcomes UN assessment mission to El Fasher
 Sharjah Airport strengthens readiness for peak winter travel season
Sharjah Airport strengthens readiness for peak winter travel season
 30-tonne UAE aid shipment reaches Gaza to combat malnutrition
30-tonne UAE aid shipment reaches Gaza to combat malnutrition
 Dubai Police crack down on e-bike violations
Dubai Police crack down on e-bike violations
 UAE condemns mosque bombing in Syria’s Homs
UAE condemns mosque bombing in Syria’s Homs
 UAE–Pakistan ties in focus during high-level talks
UAE–Pakistan ties in focus during high-level talks



