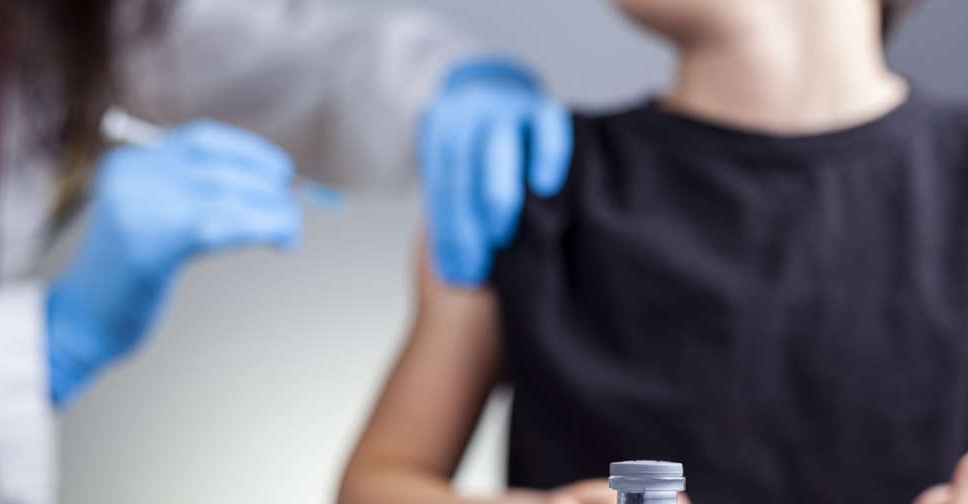
The UAE's Ministry of Health and Prevention has given emergency approval for Sinopharm COVID-19 vaccine for children aged three and above.
In a tweet, the National Emergency Crisis and Disasters Management Authority (NCEMA) said the decision was made after "the recent clinical trials and extensive evaluations, which are in line with the approved regulations".
Ministry of Health approves use of #Sinopharm vaccine for 3-17 age group #WamNews pic.twitter.com/yq8snmccJu
— WAM English (@WAMNEWS_ENG) August 2, 2021
MoHAP announced the provision of Sinopharm vaccine for the age group 3 - 17 The decision comes after clinical trials and extensive evaluations and is based on the emergency use authorization and local evaluations which are in line with the approved regulations.#TogetherWeRecover pic.twitter.com/4Y3VEMMW8V
— NCEMA UAE (@NCEMAUAE) August 2, 2021
It comes after authorities in Abu Dhabi achieved the target of vaccinating 900 children as part of a trial to study the effectiveness of the Sinopharm COVID-19 vaccine in young people.
The Department of Health Abu Dhabi (DoH) said the study examined the vaccine’s effectiveness in reducing the infection rate and severity of symptoms among target groups.
Its preliminary results will be announced at a later stage.



 World’s Coolest Winter campaign spotlights UAE entrepreneurs
World’s Coolest Winter campaign spotlights UAE entrepreneurs
 UAE set for second phase of single-use plastic ban
UAE set for second phase of single-use plastic ban
 Dubai tests drones to clean traffic signals
Dubai tests drones to clean traffic signals
 UAE leaders hail 'enduring bonds of friendship' on Bahrain's National Day
UAE leaders hail 'enduring bonds of friendship' on Bahrain's National Day
 UAE locally produces 3 oncology medicines to strengthen fight against cancer
UAE locally produces 3 oncology medicines to strengthen fight against cancer
 UAE stands in solidarity with Morocco over deadly flash floods
UAE stands in solidarity with Morocco over deadly flash floods
 Dubai mandates front number plates for delivery bikes
Dubai mandates front number plates for delivery bikes
 UAE condemns drone attack on peacekeeping base in Sudan
UAE condemns drone attack on peacekeeping base in Sudan



