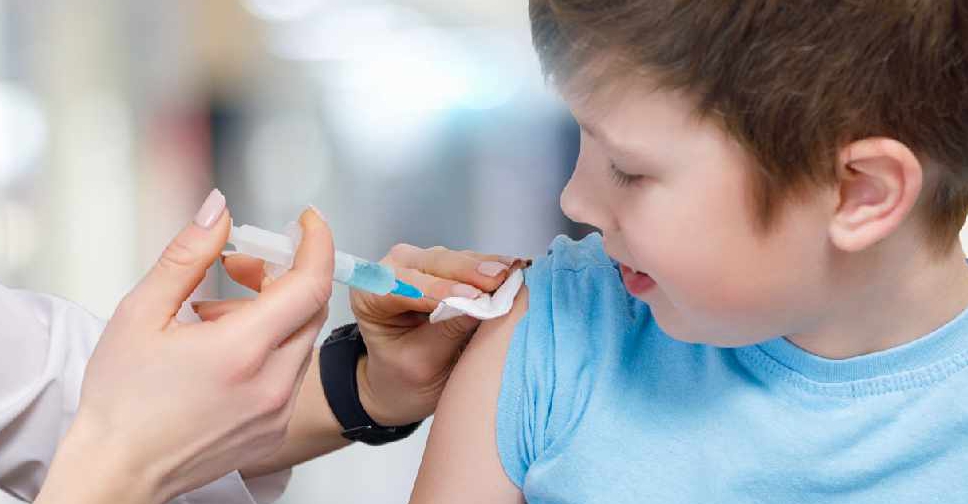
The UAE has begun to trial the Sinopharm COVID-19 vaccine in children aged 3 to 17 to study the impact on their immune system.
The study has been approved by the Department of Health – Abu Dhabi and will be conducted under the supervision of the Ministry of Health and Prevention (MOHAP).
In a series of Tweets, the Abu Dhabi Media Office explained that the study will monitor the immune response to the vaccine in 900 children of different nationalities.
Dr Nawal Al Kaabi, Chairwoman of the National COVID-19 Clinical Management Committee, who is leading the study, explained that the Sinopharm immune bridge study has been designed to ensure "children are able to receive the vaccine safely".
Each child will participate with the full consent of their parents and will be closely monitored at every step of the process.
The authorities added that it's a significant step in combating the pandemic and will support the planning process for the safe return to schools.
The study’s preliminary results will be announced "once available".
The study is in line with the UAE’s long-term recovery plan, which is based on vaccinating 100 per cent of the targeted groups by end of 2021. The study’s preliminary results will be announced once available and will support planning for the safe return to schools.
— مكتب أبوظبي الإعلامي (@ADMediaOffice) June 10, 2021



 UAE President meets French counterpart in Abu Dhabi
UAE President meets French counterpart in Abu Dhabi
 UAE, UNHCR sign deal to support Sudan conflict response
UAE, UNHCR sign deal to support Sudan conflict response
 H.H. Sheikh Mohammed congratulates 'Great Arab Minds Economics' winner
H.H. Sheikh Mohammed congratulates 'Great Arab Minds Economics' winner
 New Dubai Trade Centre bridges slash journey times to two minutes
New Dubai Trade Centre bridges slash journey times to two minutes
 UAE welcomes statements by US Secretary of State on Sudan
UAE welcomes statements by US Secretary of State on Sudan
 UAE approves Itvisma gene therapy to treat spinal muscular atrophy
UAE approves Itvisma gene therapy to treat spinal muscular atrophy
 Cloudy weather, rain forecast for parts of UAE
Cloudy weather, rain forecast for parts of UAE
 Sri Lankan President thanks UAE for flood relief efforts
Sri Lankan President thanks UAE for flood relief efforts



